Determination of Chemical Formula and the Percentage Yield of Product
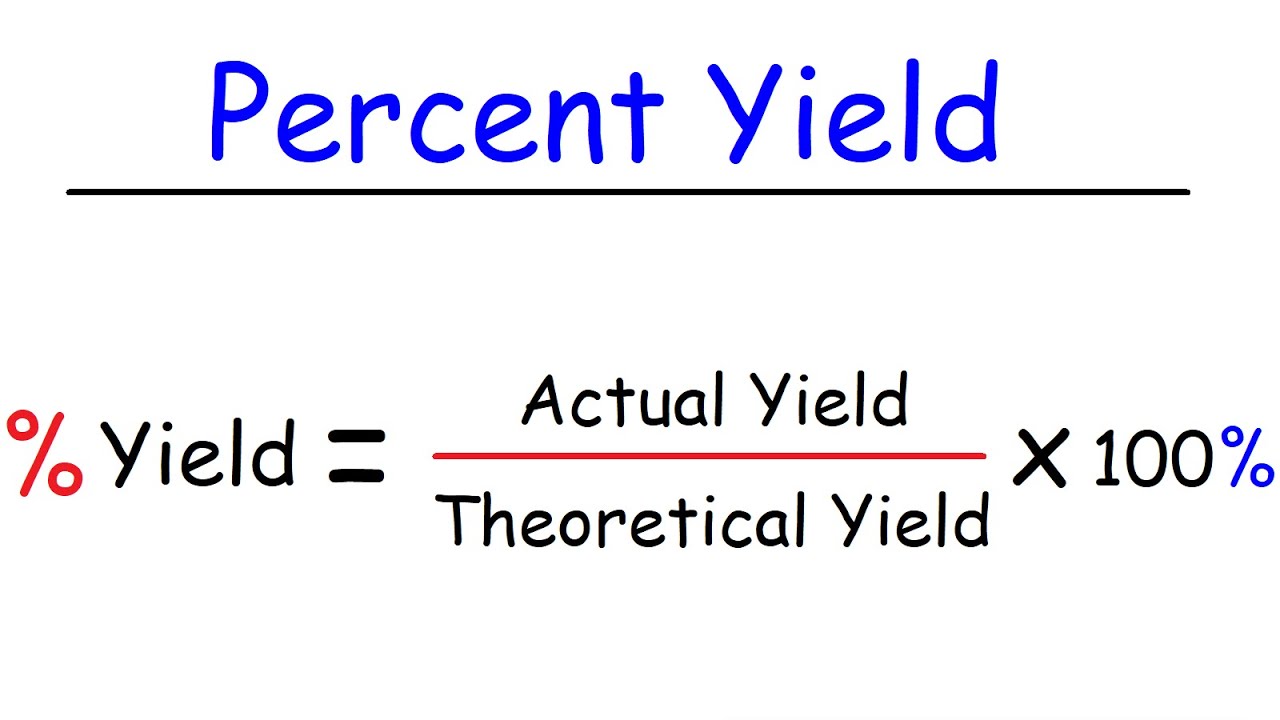
The percent yield is 100 percent if the actual and theoretical yields are equal. RmPercentagermYield fracrmActualrmYieldrmTheoreticalrmYield times 100 Q2.
How To Calculate Theoretical Yield And Percent Yield Youtube
Therefore the percentage yield of.

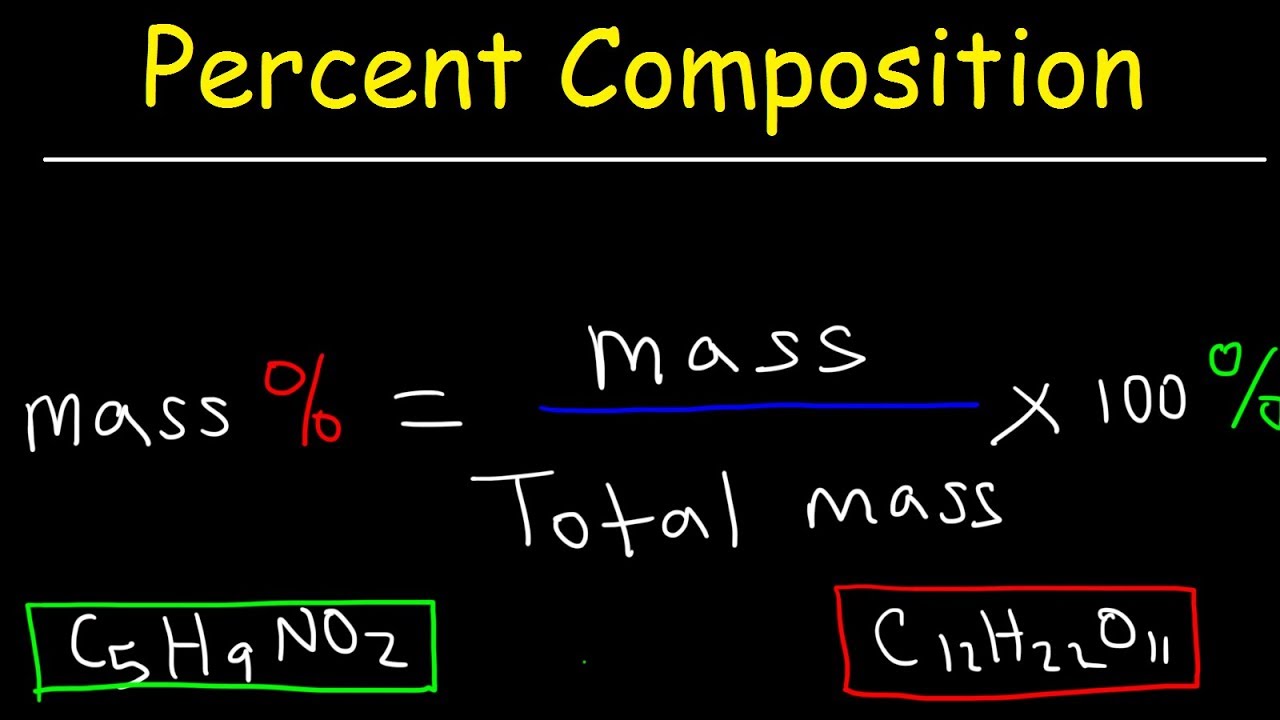
. Actual Yield Theoretical Yield X 100 Percent Yield. Ethanol is used in a 25-fold. The percent yield of the aspirin obtained from my experiment is 463 yield.
Calculate The Theoretical Yield To Determine The Yield In A Chemical. View Experiment 11 student versionpdf from CHEMISTRY PRK1026 at University of Malaysia Sarawak. 120 g 60 gmol 20 mol.
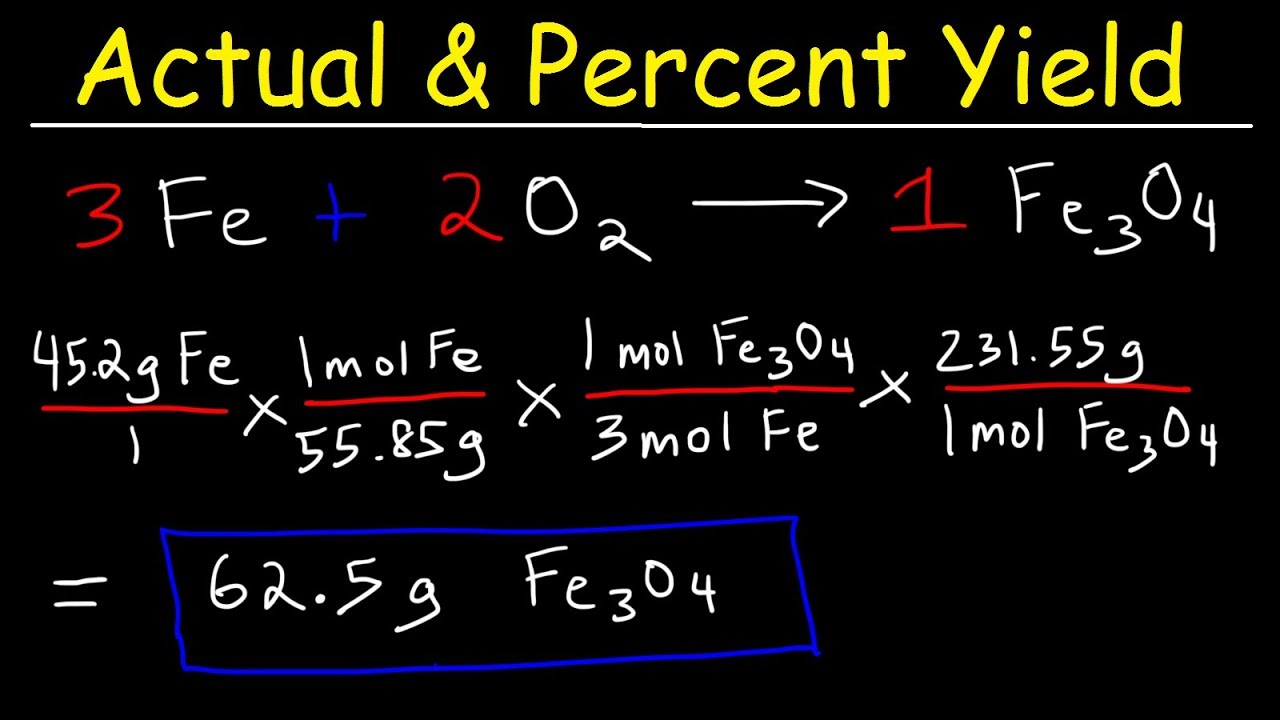
The molar amount of the reactants is calculated from the weights acetic acid. We know that according to Percent Yield Formula Percentage yield Actual yieldTheoretical yield 100. Mass of anhydrous salt used Final mass of crucible contentg after second heating- Mass of crucibleg 24891g0002g 23384g 0002g 1507g Absolute.
Percentage yield 15 20 100 75 Losing product A 100 per cent yield means that no product has been lost while a 0 per cent yield means that no product has been made. The formula for percent yield is. Substitute the values in the corresponding percentage yield formula Percentage yield Actual Yield Theoretical Yield x 100.
People also downloaded these. PERCENT YIELD The percent yield of a reaction tells us how well the reaction worked in terms of forming a desired product. The product of the zinc-iodine reaction which is soluble in methanol should now be completely transferred from the Erlenmeyer flask to the beaker.
The yield was 75. For example the chemical compound with the empirical. Its formula is.
Percent yield is used in chemistry to evaluate how successful a chemical reaction was. The molecular formula of compounds can be determined from their empirical formula and either the molar mass or molecular weight. Percent Yield Actual Experimental Yield of Product X 100.
The experimental yield divided by the theoretical yield multiplied by 100 is the percentage yield formula. Also the hypothesis was if you had the necessary components such as the theoretical and actual yield the percent yield could be found. The formula for the percent yield is equal to.
Determination of Chemical Formula and the Percentage Yield of. The Erlenmeyer flask contains only. Percentage yield 09 18 x.
230 g 46 gmol 50 mol.

How To Calculate Theoretical Yield And Percent Yield Youtube
How To Calculate The Percent Yield And Theoretical Yield Youtube

Calculate The Theoretical Yield To Determine The Yield In A Chemical Reaction Youtube
How To Calculate Theoretical Yield And Percent Yield Youtube
Comments
Post a Comment